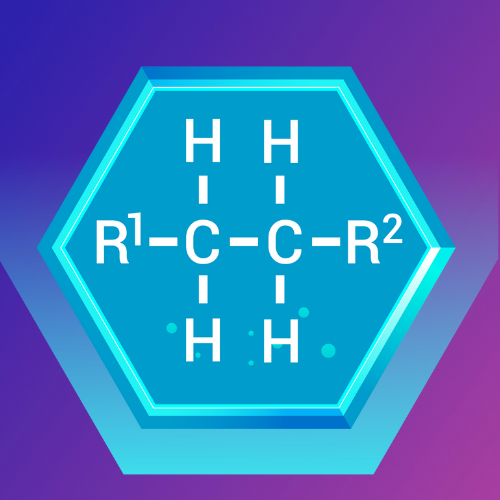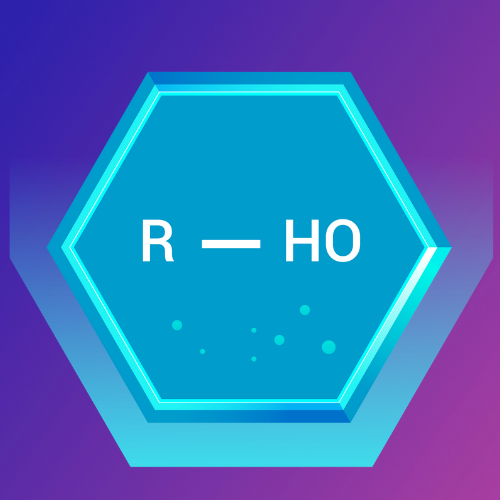
Lesson 2: Alcohols, Carboxylic Acids, Aldehydes, Ketones, and Organic Acid Anhydrides
In this post, we’ll dive into alcohols, carboxylic acids, aldehydes, ketones, and organic acid anhydrides. Along with the definitions, I’ll provide interesting real-world examples to illustrate their importance.
Welcome to Masters of the Universe!
Hello, future chemists! Today, we’re continuing our journey into the fascinating world of functional groups. Understanding these groups is crucial for mastering organic chemistry. In this post, we’ll dive into alcohols, carboxylic acids, aldehydes, ketones, and organic acid anhydrides. Along with the definitions, I’ll provide interesting real-world examples to illustrate their importance.
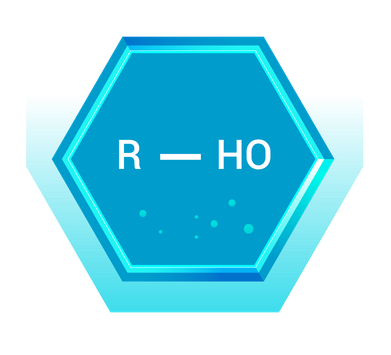
Alcohols: The Hydroxyl Group Bearers
What Are Alcohols?
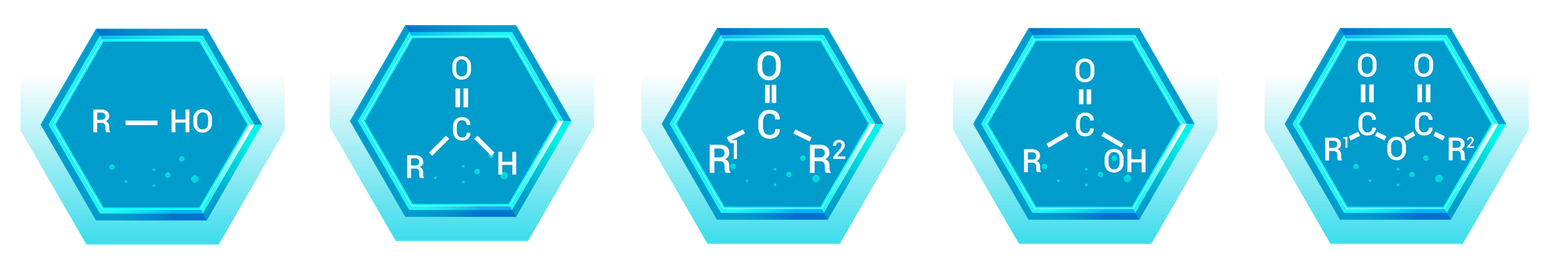
Alcohols are organic compounds that contain one or more hydroxyl (-OH) groups attached to a carbon atom. They are classified based on the number of carbon atoms bonded to the carbon bearing the hydroxyl group.
Real-World Example: Ethanol (C₂H₅OH)
- Description: Ethanol, commonly known as alcohol, consists of a two-carbon chain with a hydroxyl group attached to one carbon.
- Application: Ethanol is widely used as a beverage in alcoholic drinks. It’s also a key ingredient in hand sanitizers, and serves as a solvent and a fuel additive in gasoline (ethanol-blended fuels).
Aldehydes: The Terminal Carbonyl Group
What Are Aldehydes?
Aldehydes contain a carbonyl group (C=O) with at least one hydrogen atom attached to the carbonyl carbon. This group is typically found at the end of a carbon chain.
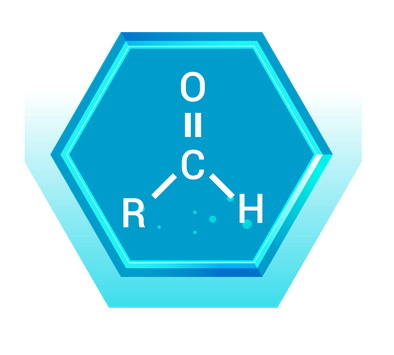
Real-World Example: Formaldehyde (CH₂O)
- Description: Formaldehyde is the simplest aldehyde, consisting of one carbon atom double-bonded to an oxygen atom and single-bonded to two hydrogen atoms.
- Application: Formaldehyde is widely used in the production of resins and plastics. It is also a key preservative in embalming fluids and has applications in disinfection and antiseptic products.
Ketones: The Internal Carbonyl Group
What Are Ketones?
Ketones have a carbonyl group (C=O) bonded to two carbon atoms. Unlike aldehydes, the carbonyl group in ketones is located within the carbon chain, not at the end.
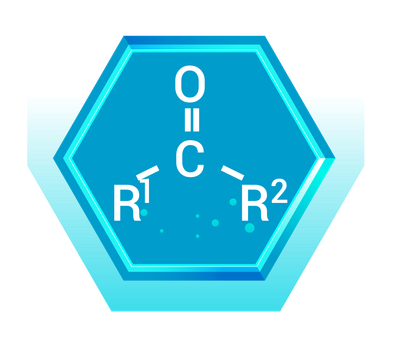
Real-World Example: Acetone (CH₃COCH₃)
- Description: Acetone, the simplest ketone, has a three-carbon chain with a carbonyl group attached to the middle carbon.
- Application: Acetone is commonly used as a solvent in nail polish removers and paint thinners. It is also used in the manufacture of plastics and other industrial products.
Carboxylic Acids: The Carboxyl Group Compounds
What Are Carboxylic Acids?
Carboxylic acids contain a carboxyl group (-COOH), which is a combination of a carbonyl group (C=O) and a hydroxyl group (-OH) on the same carbon atom. This functional group is highly polar and acidic.
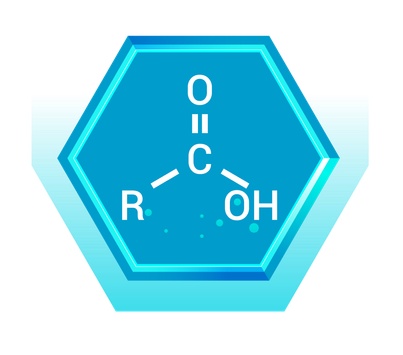
Real-World Example: Acetic Acid (CH₃COOH)
- Description: Acetic acid is a simple carboxylic acid with a two-carbon chain where the second carbon is part of the carboxyl group.
- Application: Acetic acid is the main component of vinegar, giving it its sour taste and pungent smell. It is also used in the production of synthetic fibers and plastics, and as a chemical reagent in laboratories.
Organic Acid Anhydrides: The Dehydrated Carboxylic Acids
What Are Organic Acid Anhydrides?
Organic acid anhydrides are compounds formed by the dehydration of two carboxylic acid molecules. They have two acyl groups (R-CO) bonded to an oxygen atom (R-CO-O-CO-R).
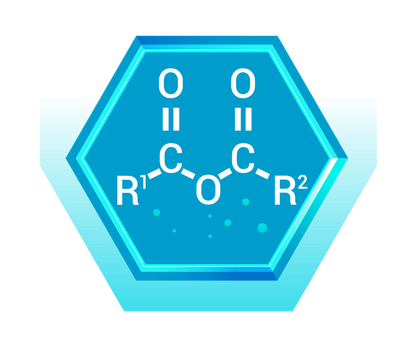
Real-World Example: Acetic Anhydride ((CH₃CO)₂O)
- Description: Acetic anhydride is derived from acetic acid, consisting of two acetyl groups bonded to an oxygen atom.
- Application: Acetic anhydride is used in the production of cellulose acetate, which is a component of photographic film and other plastic products. It is also a reagent in the synthesis of aspirin and other pharmaceuticals.
Ready to Play Level 2 in ChemEra: To Alpha Centauri?
Understanding functional groups is essential for mastering organic chemistry. Alkanes, alkenes, alkynes, haloalkanes, and arenes each play significant roles in both the chemical industry and everyday life. By recognizing these functional groups and their real-world applications, you’ll gain a deeper appreciation for the diverse and dynamic nature of organic chemistry. Now try our minigame ChemEra when you subscribe for free.
Stay curious and keep exploring the wonders of chemistry with Masters of the Universe!
Why ChemEra: To Alpha Centauri is helpful for Learning
- Interactive Learning: By combining gameplay with educational content, ChemEra: To Alpha Centauri turns the memorization of chemistry concepts into an interactive and enjoyable experience.
- Engagement: The game’s space adventure theme keeps players engaged and motivated, making it easier to retain information about chemical structures.
- Skill Reinforcement: Each level reinforces your knowledge of chemistry, ensuring you become proficient in recognizing and recalling their names and structures.
- Accessibility: Designed to be played on mobile devices, this game makes learning convenient and fun, anytime and anywhere.